1.Chemical recycling of mixtures
In biodegradable plastics, several different plastics generally need to be mixed in processing due to the requirements of physical properties24). Therefore, it is almost impossible to separate all plastic waste by type. In the chemical recycling and reuse of the mixture of polyester biodegradable plastics, the ester group is decomposed under acid or alkali conditions to form a monomer mixture. If these mixtures are separated and purified, the energy consumption and cost are considerable. However, biodegradable plastics can be decomposed into monomers under the action of environmental microorganisms, so they can be depolymerized under the catalysis of enzymes or microorganisms for biochemical recycling and reuse. Taking advantage of the fact that some enzymes act only on specific plastics (specificity), it is possible to efficiently separate only specific monomers from a mixture.
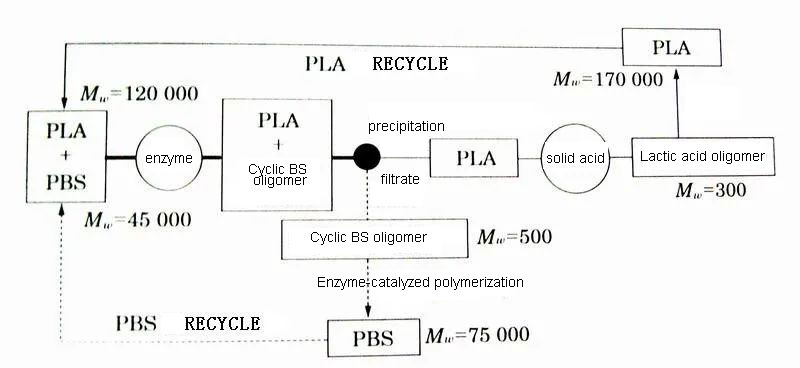
Therefore, through the combined use of enzymatic catalysis and chemical catalysis, the polymer mixture can be separated for chemical recycling and reuse. The raw material of some bottle caps used in Aichi, Japan is a mixture of PLA and PBS. PLA has a melting point of about 170°C and a glass transition temperature of about 60°C, and is a rigid plastic with excellent transparency and strength. However, due to the relatively weak impact resistance, various additives and other polymers are usually added, and then formed after mixing. Aliphatic polyesters other than PLA such as PBS are soft plastics with excellent flexibility and impact resistance. Generally, the melting point is 60-110°C, and the glass transition temperature is below room temperature. Due to high crystallinity, low transparency, and low strength Low. Therefore, there are many attempts to modify it by mixing the two. The approximate process of chemical recycling and reuse of laboratory-grade mixtures of PLA and PBS separately is shown in Figures. Under the action of lipase, the mixture solution first decomposes only PBS into cyclic oligomers. Then, the unreacted PLA is precipitated and separated by reprecipitation, and then decomposed into oligomers under the catalysis of solid acid. The cyclic BS oligomers are polymerized under the catalysis of enzymes, and the lactic acid oligomers undergo solid-phase polymerization to regenerate the corresponding high molecular weight bodies.
2.Recycling and reuse of aliphatic polycarbonate
Aliphatic polycarbonates are more resistant to hydrolysis than polyesters and are favored in various applications. Among them, P(TMC) can be used for biological materials because it can be made into an amorphous soft and tough film. P(TMC) is formed by ring-opening polymerization of cyclic carbonate monomer TMC. When a general Lewis acid is used in this reaction, a side reaction occurs in the ring-opening polymerization, and an ether bond is formed at the same time as decarbonation. However, in anionic ring-opening polymerization, decarbonation does not occur, but polymerization equilibrium occurs. If it is catalyzed by lipase, the decarbonation reaction will not occur, and the corresponding polycarbonate can be easily formed by ring-opening polymerization. In addition to ring-opening polymerization, P(TMC) can also be obtained by the enzymatic polymerization of dimethyl carbonate or diethyl carbonate with 1,3-propanediol. Moreover, P(TMC) is easily depolymerized into a cyclic TMC monomer under the action of lipase, so it can be chemically recycled and reused. A summary of these aggregation and decomposition relationships is shown in Figure.
3.Molecular design of biodegradable plastics that can be chemically recycled and reused
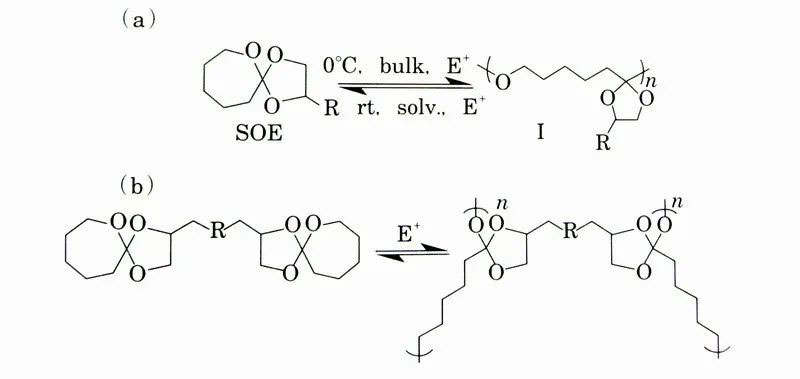
The ideal biodegradable plastic is not only required to use renewable raw materials, but also be easily chemically recycled and reused. Although it has not yet been put into use, there are still many researches on new polymer materials suitable for chemical recycling and reuse. Polymerization equilibria have been used in the design of many polymer molecules that can be chemically recycled and reused in various reports. For example, Figures show the equations for chemical recycling using the polymerization equilibrium of bicyclic ethers. At 0°C, the ether ring in SOE undergoes ring-opening polymerization to form polymer (I). Polymer (I) was depolymerized in acid-catalyzed solution at room temperature, and monomer SOE was quantitatively generated [Fig]. It has also been proposed to de-bridge the NETWORK POLYMER generated by the bridging reaction of difunctional SOE using the polymerization and depolymerization of SOE [Fig].
Attempts to recycle and reuse into the scope of molecular design considerations include the multifunctional polyester proposed by Endo et al., setting temperature and solvent conditions for polymerization and depolymerization, and the introduction of acetal bonds on PU proposed by Hashimoto et al. , recycled and reused under acid catalysis.
Biodegradable groups and polymers bound by hydrolyzable molecular bonds can obtain oligomers and polymers that can be reverted to biodegradable groups by the action of hydrolytic enzymes that are ubiquitous in nature. Hydrolyzable molecular bonds include ester bonds, carbonate bonds, and the like. The following will take the biodegradable PU that can be chemically recycled and reused as an example to introduce the research discussion at the laboratory level. PEU and PCU can be obtained by linking DUDs through ester bonds or carbonate bonds (Figure). In chemical recycling and reuse, PEU or PCU is dissolved in a solvent to generate cyclic oligomers whose main components are monomers and dimers under the action of lipase. This cyclic oligomer will rapidly repolymerize under the action of lipase in concentrated solution or bulk state to generate high molecular weight PEU and PCU.






